ABSTRACT
Background
The current standard of care for prevention of contrast-induced nephropathy (CIN) is volume expansion; however, randomized-controlled trials (RCTs) comparing interventions draw disparate conclusions. This may raise concerns about internal validity of the underlying studies. In other disease states, a high risk-of-bias (ROB) has been associated with exaggerated effect sizes, but such an evaluation is lacking for CIN prophylaxis. Ultimately, completion of this study will assist clinical pharmacists in selecting the most effective CIN prophylaxis intervention.
Objectives
To determine if ROB is associated with larger effect sizes in RCTs investigating prophylactic interventions for contrast-induced nephropathy.
Methods
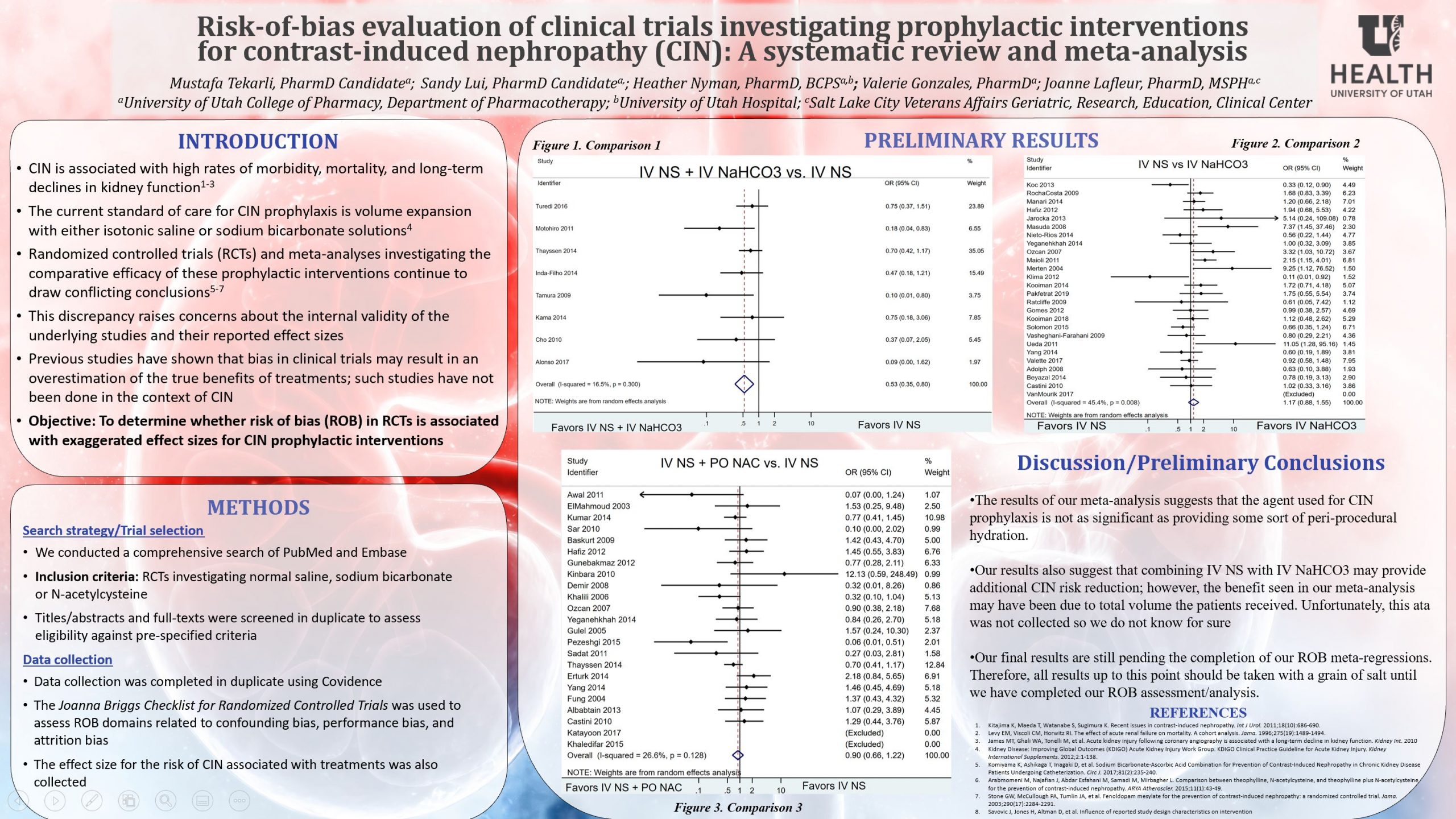
We searched PubMed and Embase to identify RCTs that evaluated renal outcomes for three CIN prophylactic treatments (intravenous [IV] normal saline, IV sodium bicarbonate, and/or IV or oral N-acetylcysteine) in patients undergoing procedures requiring contrast media. We assessed ROB using a critical appraisal tool from the Joanna Briggs Institute (JBI). We conducted title/abstract and full-text screening as well as data collection in duplicate. To examine potential publication bias and/or other small study effects, we plan to visually examine funnel plots and conduct Eggers’ tests, assuming that a sufficient number of trials is available. We will check for heterogeneity using an I2 test. Potential sources of heterogeneity, including population, intervention, and protocol differences, will also be examined in subgroup and sensitivity analyses. To test our central hypothesis that ROB is associated with exaggerated effect sizes, we will conduct univariable and multivariable random-effects meta-regressions to examine the impact of JBI ROB dimensions on the natural log of the effect sizes reported in each RCT.
Results
Our search returned 1,984 results. After title and abstract screening, a total of 209 studies remained for full-text review. Final results will be presented including coefficients for the mean change in effect sizes associated with the various ROB domains.
Conclusions
Research in-progress



Responses
The study question is very interesting. I noticed that the preliminary results are focused on effect sizes of these prophylactic interventions without the relationship of ROB and effect size. I wonder if you share insights on challenges encountered during the process which may explain why such analysis and results are still pending.
Great question! One thing we did not anticipate was having so many studies eligible for inclusion in our analysis. This lengthened the time needed for data extraction. Thereafter, the statistical analyses required for the ROB analysis has been tricky which has also slowed us down. After getting a bit of help with my statistical analysis, we should finish up soon thereafter.
Thank you for your question and your interest!
Looking good, Tafa. I see you’ve made quite a lot of progress since we last talked.
Thank you! I’m excited for us to finish it up.
Hi Taffa. What is your prediction on what the ROB analysis will reveal?
Hi Dr. Witt – great question! We predict that that trials with a high degree of ROB will be associated with larger effect sizes. Particularly with ROB domains that have to do with allocation blinding. The reason being that previous meta-analyses assessing ROB in different clinical settings have found that ROB tends to be associated with more exaggerated treatment effects. We expect our results to be similar. Thanks for the question!
Interesting. Thanks.
Thank you for your interest!
Mustafa, this is coming together. Like the others, I’m anxious to see the ROB analysis. I guess I would predict, since there is not a clear “winner” as the best therapy for CIN, other than volume, that there is likely not a high level of bias. But, I’m not savvy at all as to what drives bias, so will be anxious to see your findings!
Thank you, Dr. Keefe! We are hoping to have our ROB analysis done by sometime next week! We will definitely keep everyone posted.
Comments are closed.